
All Resources
Explore our latest resources


Viz.ai Receives FDA 510(k) Clearance for Viz™ SUBDURAL (SDH)
AI-powered Viz SUBDURAL (SDH) automatically detects subdural hemorrhage, enables effective triage and optimal care SAN FRANCISCO - July 27, 2022...
Jul 27, 2022

Viz.ai Partners with Hyperfine to Enable New MR Imaging and Workflow Paradigm
Viz.ai announced today it is partnering with Hyperfine, Inc., creator of the first FDA-cleared point-of-care magnetic resonance imaging (MRI) device,...
Jul 26, 2022
Contrast Shortage: How to move forward and continue to provide the best patient...
Explore imaging trends in the acute stroke setting across 1000 hospitals in the US to understand the impact of the...
Jul 13, 2022


March 21, 2022
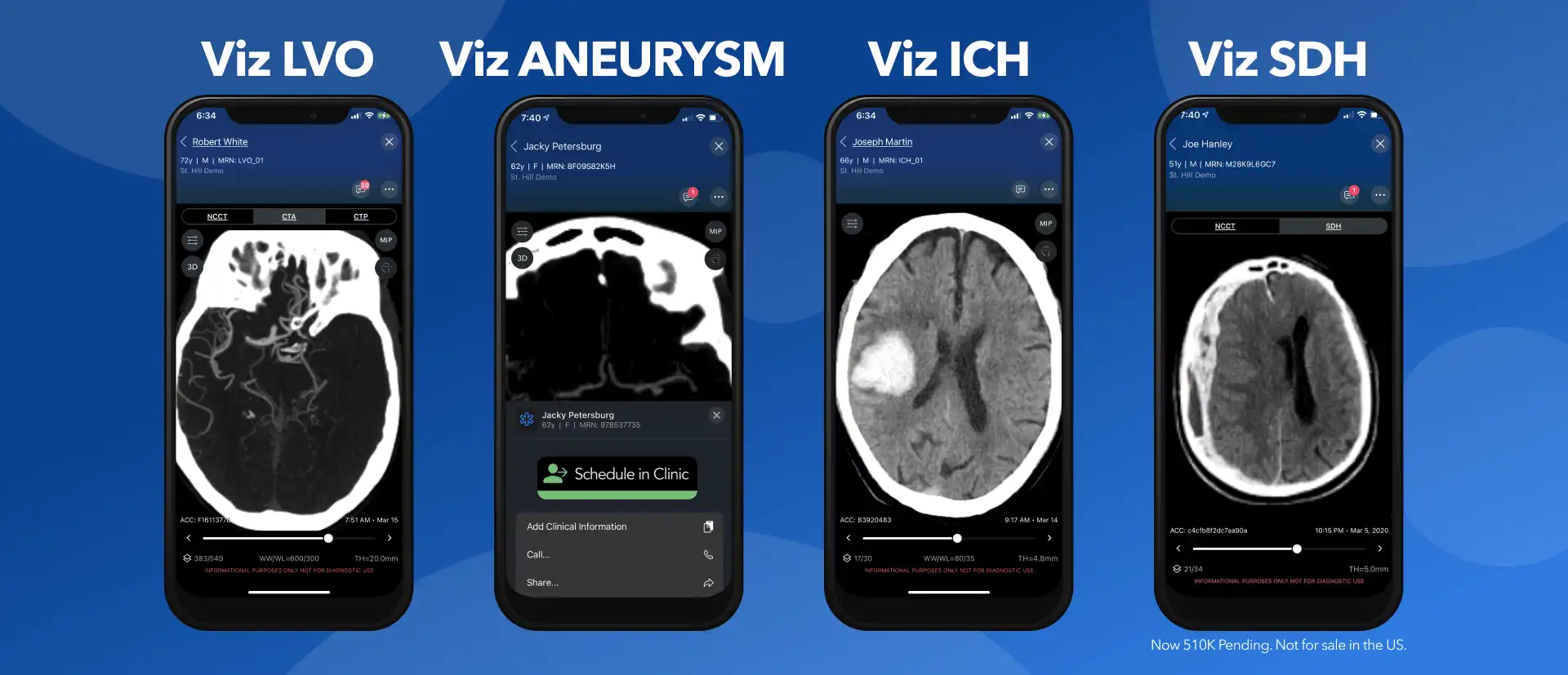
Viz.ai Neurovascular Impact Continues to Expand
Our latest blog focuses on the expansion of the neurovascular platforms with articles on the latest data from ISC 2022,...
Mar 15, 2022

Viz.ai Receives FDA 510(k) Clearance for Viz™ ANEURYSM (ANX)
Viz.ai, the world leader in artificial intelligence (AI) powered care coordination, today announced it has received U.S. Food and Drug...
Feb 24, 2022

New Data Presented at ISC 2022 Further Validates Impact of the Viz™ Platform...
Data from several new studies presented this week at the International Stroke Conference (ISC) further validates the best-in-class sensitivity and...
Feb 11, 2022