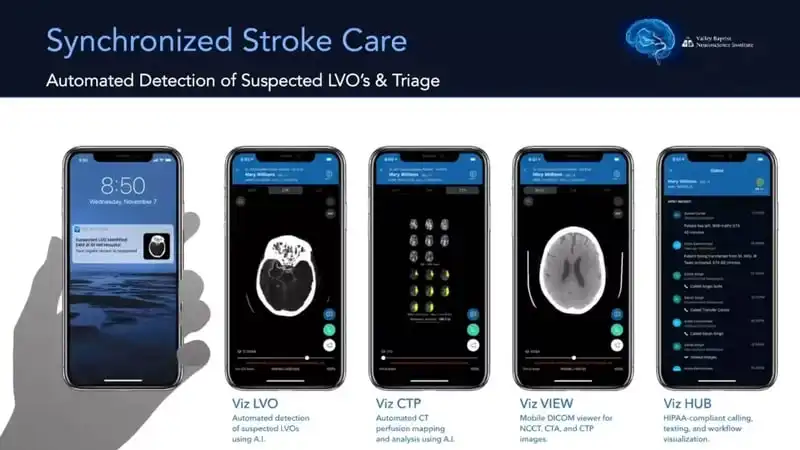
Viz.ai Collaborates with Johns Hopkins to Expedite Patient Enrollment in Brain Injury Trial
Using Viz RECRUIT software, recruitment happens in real-time, around-the-clock, accelerating treatment development for patients with intracerebral hemorrhage SAN FRANCISCO, CA...
Feb 14, 2023